This week, a team of researchers from the University of Toronto published a paper in The Lancet describing the results of a small study using deep brain stimulation (DBS) to treat severe/chronic anorexia nervosa. Major news outlets, including the BBC, reported on the findings. A few people emailed and messaged me asking me to do a post about it (which is cool! I love it!). So here it is.
DBS is a surgical procedure that involves implanting an electrode that delivers electrical signals to the brain. DBS is used to treat Parkinson’s disease and other movement disorders with good success, and has recently been implicated in the treatment of OCD and depression as well. (This is a pretty good video explaining how DBS works for movement disorders. There’s lots of information online about how DBS works, so I won’t go into detail here.)
This is not the first time that DBS has been used to treat anorexia nervosa patients (and I actually remember hearing about this when I was in undergrad, a few years ago). There have been two case reports and one study of acutely ill adolescents with the disorder. This study, however, assessed DBS in six treatment-refractory AN patients. Since this is a phase 1 pilot trial, the major goal was to evaluate the safety of the procedure and identify side effects.
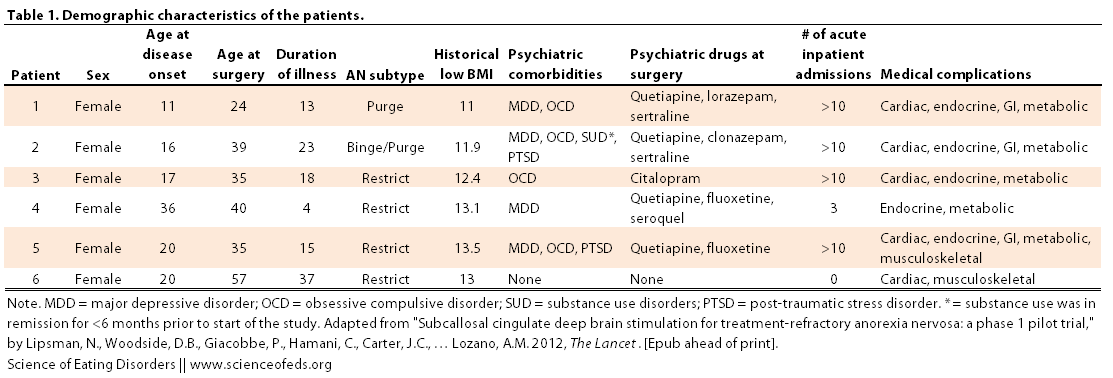
Patients had to between the ages of 20-60 and show a pattern of chronicity or treatment resistance. I’ve included the inclusion and exclusion criteria and a table describing the demographic characteristics of the patients below (Click on image to enlarge).
(Just FYI: BMI values are mentioned.)
Inclusion criteria
- Female or male patients aged 20–60 years
- Diagnosis of anorexia nervosa, restricting or binge–purging subtype, as defined in the Diagnostic and
- Statistical Manual of Mental Disorders (DSM-IV-TR)
- Chronicity or treatment resistance shown by some orall of:
- A pattern of 3 years’ duration of relentless unresponsiveness to repeated voluntary hospital admissions, characterised by failure to complete treatment or immediate weight relapse after treatment
- A pattern of increasing medical instability, accompanied by refusal to participate in or a pattern
- of poor response to intensive expert treatment and increasing medical acuity, lasting at least 2 years and including at least two episodes of involuntary feeding
- A pattern of chronic stable anorexia nervosa that has lasted at least 10 years
- Able to provide informed consent
- Able to comply with all testing, follow-ups, and study appointments and protocols
Exclusion criteria
- Any past or present evidence of psychosis
- Active neurological disease such as epilepsy
- Alcohol or substance dependence or abuse in the previous 6 months, excluding caff eine and nicotine
- Any contraindication to MRI or PET scanning
- Likely to relocate or move during the study’s 1-year duration
- Body-mass index less than 13
- Presence of cardiac arrhythmias or other cardiac, respiratory, renal or endocrine disorders, as a result of
- anorexia nervosa or not, that will result in substantial risk from a surgical procedure
- Pregnancy
(Honestly, I’m not sure why patient 4 was included…)
Lipsman et al selected a region called the subcallosal cingulate as a target for DBS. Their reasons were as follows:
- “imaging studies show similar patterns of activity in the subcallosal cingulate region and in its afferent and efferent projections in patients with anorexia nervosa as are seen in patients with depression;”
- “anorexia nervosa and mood and anxiety disorders are often comorbid, with similar anatomical structures and circuits implicated”;
- “subcallosal cingulate DBS improves symptoms in patients with treatment-refractory depression and reverses cerebral metabolic abnormalities in dysfunctional limbic circuits.”
MAIN FINDINGS
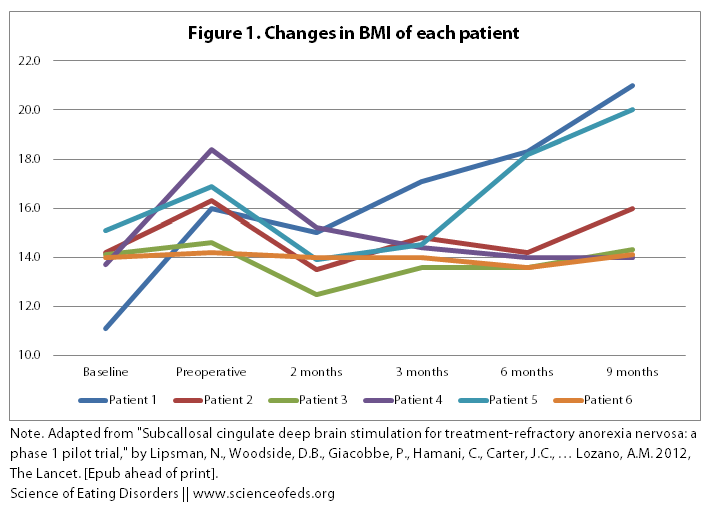
1. BMI. Below is a table and a graph describing the BMI changes of the six patients during the course of the study. “Baseline” was taken as the average BMI in the 5-7 years prior to the study, assessed through doctors’ notes, an interview, and the patients’ diaries.
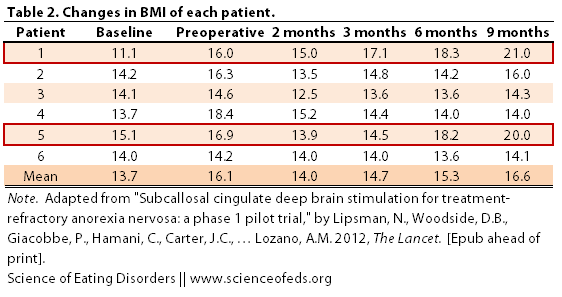
The BBC reported that “three people were able to gain weight and had improvements in their overall mood after undergoing the procedure.” But, when you look at the graphical representation of the table above, one thing becomes immediately clear: what three people? I only see two people with significant improvements in their BMI (Patient 1 and Patient 5).
Also note that the preoperative BMI was substantially higher than the baseline BMI (from a mean of 13.7 to 16.1). This is because patients 1 through 5 were attending inpatient treatment immediately prior to the onset of the study, which resulted in some weight gain. There was no mention of whether this treatment, or any other treatment (psychotherapy, nutritional counselling) was continued or initiated during the following 9 months post operation. (Only that no changes were made to medications in the three months following the procedure.)
I only found this bit of information in the press release:
Furthermore, four of the six patients also experienced simultaneous changes in mood, anxiety, control over emotional responses, urges to binge and purge and other symptoms related to anorexia, such as obsessions and compulsions. As a result of these changes, two of these patients completed an inpatient eating disorders program for the first time in the course of their illness.
This is a confounding factor. How do we know the effects were due to DBS and not the inpatient treatment? How do we know DBS helped them complete treatment, given that 4/5 patients receiving inpatient treatment experienced weight gain as a result of it?
In fact, because there was no control group, we really can’t comment on efficacy at all. All we can say is that DBS has potential, it is promising. There’s nothing wrong with that, and indeed, the intent of the authors was to “offer this procedure only to patients who might be expected to continue with a chronic illness or die a premature death because of the severity of their illness.” It would be unethical to withhold treatment we know has some efficacy in favour of an experimental procedure. But, it means that we need to be cautious in our response to the findings.
2. Mood. On the whole, there were improvements in mood, as assessed using various depression, anxiety, and obsessive-compulsive questionnaires. The two patients that experiences the most improvement in their BMI also showed the most significant reduction in scores on the various questionnaires (some patients showed no improvements at all).
There are two problems here, though:
- Lipsman et al only show the psychometric data up to the sixths month post-op. What happened to data for the ninth month? The study followed-up on the BMI for 9 months after the operation… what about mood assessments? That’s fishy. It makes me wonder if the data wasn’t ideal and so they just didn’t include it in the paper (which happens all the time, unfortunately).
- Regression toward the mean (which is true for the BMI, but less so given that baseline BMIs were supposedly the average of the last 5-7 years). Basically, most people seek treatment when they are at their sickest. It follows, then, that the only way forward is to improve. Perhaps the improvement in the scores is not due to the DBS (and we can’t tell, because there’s no control group) but merely to the fact that the scores observed prior to the operation were exceptionally poor, even for these patients, and usually they score somewhere in the range observed 6 months after the operation. Until we do controlled studies, we don’t know.
3. Brain imaging. Interestingly, the changes observed in the patients mimic the changes seen in treatment-resistant depression patients treated with DBS. In addition, there was a decrease in metabolic activity in the insula, a region that has been heavily implicated in current models of anorexia nervosa. There was also an increase in metabolic activity in another brain region, the parietal lobe. However, the clinical correlates of these changes (as far as I can tell) are not completely clear (though there are tons of pet theories and ideas).
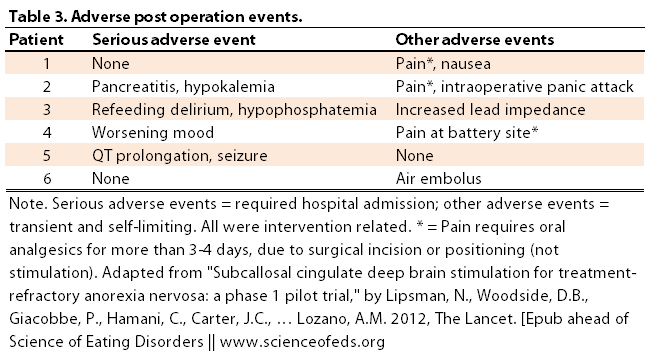
4. Adverse events. Below is a table of the adverse events experienced as a result of the operation:
IMPLICATIONS & FUTURE RESEARCH
There are tons of implications, but I’ll just briefly cover some (and leave the rest for the comments section). Well, first: it is promising. Given how difficult it is to treat chronic and treatment-refractory anorexia nervosa, I welcome any new promising treatments.
The fact that this is not something that can be easily scaled up (few places in the world do DBS surgeries, Toronto being one of them, and then there’s the issue of cost and insurance coverage) might not be a huge problem if it is used only in a limited number of very treatment-refractory cases. But our goal should continue to be: to treat eating disorders at the earliest possible point and to focus on non-invasive treatment approaches that can be easily scaled-up. If this works, I think it should be a last resort treatment.
In the discussion, the authors noted that for these patients DBS might improve their mood and behaviours to a point where they can engage in standard treatments “that are heavily weighted toward psychotherapy.” The idea, then, is not that DBS itself is the only treatment or cure, but that it enables the patient to finally engage in treatment (“a food-in-the-door technique”). This makes perfect sense to me, and I think it is a worthy goal in its own right.
I’m not well-read on the use of DBS in neuropsychiatric disorders. If there’s clear evidence it works, that’s good, even if its effectiveness is limited to specific cases. Still, my initial hesitation has to do with the fact that in Parkinson’s disease, we have a clear understanding of what’s going wrong (the death of dopaminergic neurons in the substantia nigra), though even there there’s controversy about what DBS is actually doing and how it works. For complex neuropsychiatric disorders like OCD, depression, and anorexia nervosa, we simply can’t point to a specific brain region and go “Aha!” Still, I realize we don’t know how many of the drugs out there on the market work, so, perhaps my hesitation is not justified.
Ultimately, though, I think we need to be extremely cautious with our initial reactions to the findings in this study. I’m a bit worried about the media attention this small pilot study is receiving. Let’s reserve the excitement and optimism for the future, when we know the results of blinded, sham-stimulation controlled experiments.
References
Lipsman, N., Woodside, D., Giacobbe, P., Hamani, C., Carter, J., Norwood, S., Sutandar, K., Staab, R., Elias, G., Lyman, C., Smith, G., & Lozano, A. (2013). Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial The Lancet DOI: 10.1016/S0140-6736(12)62188-6
I read the sample study were females. I wonder if estrogen and testosterone would be influential variables in the study. Also said that their OCD, PTSD, Depression were in remission for about 6 months prior so I wonder if that skewed the results. However, while this sounds like a great alternative to other methods, the end results may not outweigh the short term effects such as those who had endocrine, skeletal, cardiac, and other metabolic complications. My opinion: this method should be considered still in the early stages with room for improvement before attempting it.
(I replied on FB, but just for others). The 6 months remission was just for SUD (substance use disorder) for one patient (because substance use was an exclusion criterion). The psychometrics showed, overall, decrease in OCD, depression, and anxiety scores (but of course, a lot of variability between participants). Yes, this is VERY early, we need blinded sham-controlled studies to really know if there’s an effect or just placebo (or other things, like inpatient treatment). The hormone point is an important one, I wonder how it would play a role, too.
I’m very surprised they continued inpatient treatment after DBS. I would hender a guess that they might have thought given the patients’ previous refractory response to inpatient treatment that any effect seen after DBS could be attributed to DBS (which is, of course, a fallacy). It’s more likely that DBS acted as the permissible factor by “resetting” certain neural circuitry, allowing dugs/impatient treatment to take effect. Just my 2 cents.
I also wonder if the change in attitudes towards eating is a secondary effect to (or made possible by) improvement in mood, since subcallosal cingulate DBS has been shown to improve symptoms in depression before, or a primary effect of DBS itself. In terms of end goal this distinction doesn’t really matter, of course.
Hopefully they’ll follow up.
A bell curve would be nice with a large enough sample
Well, my problem is that none of this was disclosed. I mean, how did this get past the reviewers?? There’s NO mention of treatment in the 9 months post surgery and no mention of the treatment prior to the surgery, either. I mean, what.. Only the news release mentioned it!! NOTHING anywhere in the paper.
Yeah, the DBS might be a “foot-in-the-door” thing as they mentioned in the discussion, but.. yeah, not enough evidence for that, either.
Yeah. I didn’t read the depression DBS studies but talking to a friend today, she said none of those studies had sham-controls. So, how much can we really attribute to the DBS… too many studies without controls!
a mean of 6 patients does not really show a great deal of deviance for error one way or the other.
Yeah, I mean it is just to test whether there’s any potential and see the potential side-effects. Basically, it is a decent (ish) pilot, but, the media attention it is getting is disproportionate with the findings.
OK, Tetyana, my thoughts as promised. . .
1. To provide some background as to the MDD research – The study authors state that their rationale for implanting the electrodes the subcallosal cingulate has been shown “to improve treatment-refractory depression and reverses cerebral metabolic abnormalities in dysfunctional limbic circuits” However, the study cited really does not support this statement(Mayberg et al., 2005)
*Specifically this 2005 investigation was a similar small feasibility study including 6 patients with treatment resistant MDD (very strict criteria). There was actually some sham stimulation during the calibration period (5 days) to rule out an immediate placebo effect. However, a placebo effect (i.e. improvement in depression scores relative to baseline) would be likely present to some degree after a major surgery proposed to improve treatment. Also, the sample size was insufficient to allow for accurate comparison between responders and non-responders though after 6 months, 3 of the 6 patients met criteria for depression remission (i.e. HDRS-17 score of less than 8.) Interestingly, stimulation was turned off for a period of 4 weeks in the patient that had shown the best clinical response and there was no relapse into MDD. The authors acknowledge the lack of placebo control, appropriate sham stimulation, small sample size, medication use and variance in electrode placement as limitations in their conclusions.
* In 2012, a larger follow-up multi-site study (Lozano et al., 2012) was conducted in this patient demographic (n=22). Interestingly, 57% patients showed a 50% reduction in depression scores,relative to 48% and 29% at 6 months, and 1 year, respectively. Again, the authors acknowledged that a placebo effect could not be ruled out (no sham or placebo arm) and as the HDRS-17 (primary outcome measure) only accounted for some depressive symptoms, use of other rating scales (i.e. BDI, HDRS-29) might have yielded different results.
** My primary concern is that this preliminary study was conducted on treatment resistant AN patients who likely are vulnerable to medical instability, weakened immune system and complications. As the treatment resistant MDD DBS studies are still in a “phase I/II” proof of concept phase, it would have been nice to see more research in this area before testing this treatment in a such vulnerable population with multiple (possibly confounding) comorbidities.
2. I still think “baseline BMI” should not have been included, as this information was 5-7 years old and represented the patients’ lowest weight. There is the underlying assumption that this represents the “natural history” of the disorder, but it is not clear as to whether the patients consistently maintained this low body weight for a length of time or whether it represented a short transient drop in weight.
*The pre-operative BMI is more relevant as an immediate measure. The inclusion of BMI baseline data makes it seem as though most participants experienced dramatic improvement – which as Tetayana points out, is not supported by the data in the paper itself.
3. Also, it is unclear as to why the questionnaire/interview data for the 9 month period is not shown or even mentioned in the paper. This IS fishy as Tetyana points out – and it is unclear as to whether patients were receiving additional treatment (if any) during the 9 month follow-up period. . .
*The press release reports 2 of 6 patients finished IP treatment and the article reports that 2 of the 6 patients achieved significant weight gain. I cannot help but wonder if the former 2 individuals were the same 2 patients reported in the article – this would suggest that treatment is a possible confounding variable.
*Intensive treatment (i.e. day patient or inpatient treatment), at least in Ontario,is terminated upon attainment of a BMI of 20. Weight gain in such severe patients could easily take 6 months. Their eventual return to the community (i.e. at 9 months) might explain the absence of 9 month data from the paper and indicate a worsening of mood/anxiety symptoms due to the absence of intensive support.
5. “Our results, based on composite scans from all six patients, show that DBS of the subcallosal cingulate area in patients with anorexia nervosa produces decreased subcallosal cingulate and medial frontal activity and increased parietal activity, similar to the changes seen with stimulation of this area in patients with treatment resistant major depression.”
* This replication of PET data is at least promising. . .
5. “The number of patients is too small to speculate about possible
predictors of treatment response or the characteristics of responders compared with non responders, both of which remain questions for future investigation.”
*And yet, both the media and study authors (i.e. in press interviews) promote this as a promising intervention for treatment-resistant anorexia nervosa. As Tetyana has stated and I will emphasize, that is NOT what this study was designed to prove – this research merely suggests that DBS treatment is both tolerable and safe in patients with severe AN.
Yeah. I agree. Basically, this study suggests that this treatment has potential, but a lot more needs to be done to figure out just *how* much potential it has.
I’m not sure about your concern with the “medical instability, weakened immune system, and other complication” though… why do you think this is a confound? How could treatment resistant AN not include that, to some extent, anyway.. I’m not sure I think of it as a confound.
I think I was a bit unclear. . .
1. The medical instability, weakened immune system and medical complications concern me just because there is more risk to doing (any type) of surgery on these patients than on relatively physically healthy patients with PD or MDD.
* But if it is a life or death matter, I suppose the possible benefits outweigh the risks.
2. My issue about psychiatric comorbities (especially depression) was that the premise of the study was that this is a possible treatment for treatment-resistant AN – but these patients didn’t just have AN. . .
There is already some preliminary evidence for the efficacy of this EXACT treatment (i.e. same electrode positioning) in alleviating MDD. So, in theory, the improvement could simply be an outcome of treatment for co-morbid depression as opposed to AN.
Of course, this doesn’t mean DBS isn’t useful for AN, it just might mean that this treatment would be less effective in cases where depression wasn’t a comorbidity
It’s just hard to isolate exactly what is causing the effect when there are multiple psychiatric illnesses present. . . but again, this study really wasn’t meant to do that anyway, so perhaps this point is irrelevant.
Yes, good points. I think the benefits of DBS probably outweigh the risks of staying ill–since staying ill means a lot of health problems, including death.
Good point on number 2 (particularly that perhaps it won’t be so helpful in patients without comorbid MDD)?? It is also hard to tell how much of these comorbidities are due to the AN…
Ok so here it is… I was one of those patients in the study… I do no appreciate your questioning whether treatment t was necessary and if that is the only way/reason it helps. I had been in hospital most of my
Life the past 10 years battling anorexia and being so ill I was in ICU… I had tried every program available including tgh many times… There really was no other choice. DBS or death… And soon. So as there is no “control group” that you can see, I feel
It is enough evidence from our pasts, about how treatment was a failure. I was one who DID finally finish a program and get to a healthy weight for the FIRST time… DBS helped me get there. It allowed me
To actually engage in treatment for the first time. DBS on its own is NOT a cure, intensive treatment afterwards is still needed!
I’m so glad that the world is learning about it… But a little annoyed at how much a page that I have always lived to look to for Interest in my Illness, is not thankful for this new way to help people who really have no other hope left…
Steph, thank you for your comment. I’m really glad to hear that DBS has helped you. I don’t doubt it has helped you.
I think you’ve misunderstood my criticisms. I didn’t question whether the treatment was necessary. Not at all. What I questioned was why it wasn’t disclosed in the paper. This is a huge oversight from the authors perspective. It must be included so that readers know the protocols that were followed. It is misleading to exclude other treatments that were going on, in addition to DBS, because that overemphasizes the benefits of DBS. It is absolutely great if DBS enables individuals (you) to finish treatment. That’s worthy in its own right, as I stated in the post. But, my point is that it still need to be disclosed.
I am critical of the study protocol itself (why was the 9 months point excluded, why wasn’t treatment disclosed), not at ALL critical of using or trying to use this approach for treatment. If future, larger, blinded sham-controlled studies show that it works, that’s AMAZING. Truly amazing.
I do not want to push this potential treatment aside because this is one of the first studies to attempt to use it and, because it is a pilot study, it is small and uncontrolled.
No. My criticism is that the media reaction (and the author’s claims) are beyond the scope of what the paper has actually shown. Again, that doesn’t mean it doesn’t work, or that it doesn’t have potential, or that it didn’t work for you. Not at all.
I wrote:
“Well, first: it is promising. Given how difficult it is to treat chronic and treatment-refractory anorexia nervosa, I welcome any new promising treatments.
[…]
In the discussion, the authors noted that for these patients DBS might improve their mood and behaviours to a point where they can engage in standard treatments “that are heavily weighted toward psychotherapy.” The idea, then, is not that DBS itself is the only treatment or cure, but that it enables the patient to finally engage in treatment (“a food-in-the-door technique”). This makes perfect sense to me, and I think it is a worthy goal in its own right.”
I appreciate that you took the time to leave a comment. Thank you.
I hope you continue to enjoy the content of the blog in the future.
Cheers,
Tetyana
Fascinating post! I wonder if the variable results from the participants could have something to do with the very very specific location of the electrode. I remember hearing a talk on DBS for depression and tiny differences in location made a big difference in the outcomes.
Also, what stimulation frequencies were used?
Yeah, I thought about that (re: slightly different brain regions), or maybe a completely different brain region altogether?
Regarding the stimulation frequencies and amplitude: “We started all patients at an amplitude of 3·5 V, pulse width 90 μs, and frequency 130 Hz. Stimulation settings were changed on the basis of patient and physician feedback. Frequency and pulse width remained unchanged for the duration of the study, with amplitudes ranging from 5 to 7 V in all patients.”