In 1967, Routtenberg and Kuznesof reported a very peculiar phenomenon in rats:
They discovered that when rats were on a restricted feeding schedule (1 hour per day in their experiment) and had free access to a running wheel, their food intake was significantly lower than in control rats, which were on the same feeding schedule but without access to a running wheel. This discrepancy between increased running activity and decreased food intake caused substantial body weight loss, and if rats were not removed from the experimental setup timely, they would eventually die of starvation. This model, later named the activity-based anorexia (ABA) model, is one of the most widely used animal models for the study of anorexia nervosa (AN). (Source)
Of course, rats are not humans. Nonetheless, animal models of anorexia nervosa can inform us of some of the underlying neuropsychological and physiological influences and consequences of anorexia nervosa.They can help us understand what leads to and happens as a result of self-induced weight loss, restriction, and hyperactivity.
The benefit of using the ABA model (all models, actually) is that they enable us to control for environmental factors and individual differences that may muddy our interpretation of human data. They can also help us pinpoint physiological and behavioral alterations that result from running a chronic calorie deficit. Indeed, the drive to exercise (and in the case of rats, the drive to run) during starvation may be one factor that has been preserved across species–from rats to humans.
However, what we find in rats shouldn’t be taken as a direct reflection of the human condition as an animal model cannot represent every component of a mental disorder. This, by the way, is one of errors that Bergh & Sodersten–the individuals behind the Mandometer treatment–make: They continuously ignore the fact that the ABA model, though important, is far less complex than the human condition of anorexia nervosa.
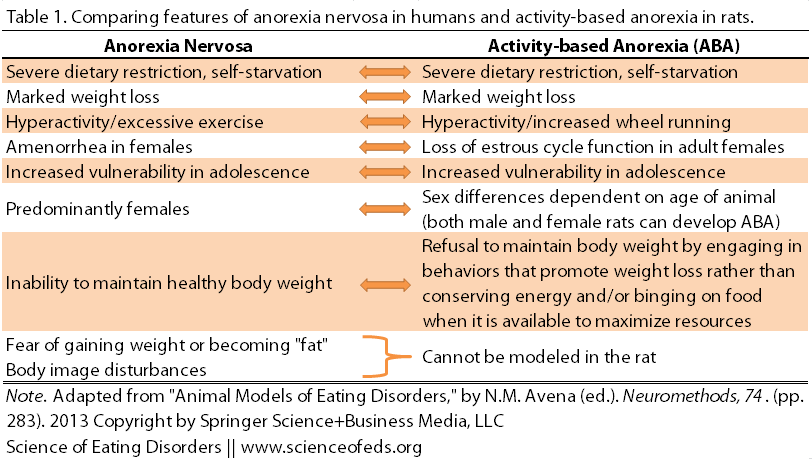
Still, there are many similarities between the ABA model and the human condition of anorexia nervosa:
But there are also differences: For example, the rats don’t voluntarily restrict their food intake (though they do eat less as their running increases), and not every anorexia nervosa patient displays excessive exercise. Moreover, the psychological components of AN simply cannot be modelled in a rat; we can’t ask a rat how she feels about her body.
Despite these differences, the neurochemical and physiological changes that occur in ABA may enable us to gain a better understanding of anorexia nervosa. For example, ABA rats that are running show “reward-like” brain responses, perhaps like the self-reported relief that exercise can provide for patients with AN.
Much of the research using the ABA model, however, has focused on male rats. In and of itself, this isn’t weird or unusual in the research world. It is a problem that has a name: it is called the “male sex bias.” (More here.) Anorexia nervosa, however, predominantly affects females, so it is even more important to study the ABA model in female rats as well. Especially given the whole reproductive cycle thing. (We have the menstrual cycle, they have the estrous cycle.)
So, in 2003, when Dixon, Ackert, & Eckel sought to examine the development and recovery from activity-based anorexia, they used female rats. And in fact, they were particularly interested in understanding how hormone levels may influence food intake patterns in ABA rats during the “recovery” stage. (Hence, using female rats is kind of important.)
The researchers had two virtually identical groups of rats. Both groups had limited access to food; the only difference was that one group had access to the running wheel (and thus developed ABA) and the other group didn’t (and thus did not develop ABA, though these rats did lose some weight as a result of the restricted eating).
Following the standard protocol for inducing ABA, the researchers gave both groups of rats free access to food. Dixon and colleagues wanted to examine the effects of freely-available food on food intake patterns and body weight gain amongst the animals. That is, what happens during the rats’ recovery from ABA?
What they found was that the rats ate a lot–in jargon terms, they exhibited hyperphagia–but the effect was much more pronounced in the group that had access to the running wheel. The runners also continued to display high levels of food intake even AFTER weight was restored and their estrous cycle was re-established.
Like the rats, abruptly quitting excessive exercise made me feel ravenous, and I continued to feel weak and hungry despite recovering to my pre-ED weight. Why couldn’t I just eat normally and feel okay? Why was it taking so long for my body to respond to the change? I took solace in discovering that my drive to persistently over-eat may have been due to a preserved adaptation, likely influenced by neurobiological changes.
WHAT BRAIN CHANGES MIGHT OCCUR AS A RESULT OF RUNNING A CALORIE DEFICIT?
The authors suggest that there may be a disruption in brain hunger and satiety signals that persist even after weight restoration. Indeed, some studies have indicated that the impact of food restriction, coupled with exercise, alters signals in the nervous system that impact food motivation and reward. Though the exact mechanism by which these signals regulate food intake and exercise remains unclear.
This makes sense to me. The brain may interpret reduced caloric intake and excessive exercise as an extremely stressful, famine-like atmosphere. In order to survive, brain signals change to encourage excessive food-related motivation and consumption. The longer this state exists, the more likely those changes will persist too–sort of like preparing for long-term famine. So when the situation changes–in the case of the ABA rats, food becomes freely available–it may take the brain some time to re-adapt to the new situation. It is as if the nervous system is skeptical: Is this food going to last? What if it doesn’t?
In other words, brain signalling likely persists as if the body was in a starvation state, even during weight recovery. For some time, anyway–not forever.
Of course, we cannot forget that many maladaptive behaviors have been learned as a consequence of having an eating disorder, and these behaviors may also interact with starvation-induced overeating. Learned fear and anxiety around food intake (and/or weight gain) may equally influence how we react to food, and override any possible alterations in hunger or satiety signalling. This may be one reason why not every individual recovering from AN experiences starvation-induced overeating, for example.
TAKE HOME MESSAGE
Clearly, we can learn more about anorexia nervosa (and other conditions) from animals, but these models are not a direct reflection of the human experience, and we must keep that in mind when interpreting study findings. They are, afterall, models.
Still, for me, these carefully-controlled studies, provide me with comfort that perhaps I’ve incurred similar disruptions in my ability to detect hunger and satiety. And that knowledge makes me feel better when I cannot figure out the appropriate amount of food to consume because I know there are neurological reasons for that. (Some of these) reasons have likely served us, and our animal cousins, well throughout evolution. Such evidence gives me a bit of self-forgiveness and understanding, and I hope it provides a bit of the same for others as well.
References
Dixon, D.P., Ackert, A.M., & Eckel, L.A. (2003). Development of, and recovery from, activity-based anorexia in female rats. Physiology & Behavior, 80 (2-3), 273-9 PMID: 14637226
Hillebrand, J.J., Kas, M.J., van Elburg, A.A., Hoek, H.W., & Adan, R.A. (2008). Leptin’s effect on hyperactivity: potential downstream effector mechanisms. Physiology & Behavior, 94 (5), 689-95 PMID: 18495181
Just…brilliant. Thank you, as well, for sharing the keen self-observations in reaction to this study. This helps me to somehow “deal” with the difficulties of food regulation in my quest for anorexia recovery.
This explains much about the “over-shooting” possibilities as the “lizard” brain part of our entities fears for potential future famine periods and why “hunger” can persist past satiety of the stomach.
Thanks, Donna! I’m SO glad you feel some comfort by this :).
In the process of editing this post and adding some stuff, I found out that ABA rats don’t eat as much as you think they would in the 1/2hrs they are given access to food. Basically, they are not “stuffing themselves” in that time period and they seemingly voluntarily decrease their intake as they increase their running. What gives? Do you have any ideas about that and why that’s not the case when they are given free access to food? I don’t know a lot about ABA, so I’m wondering if you know/have any ideas? (I’m sure it is in the lit somewhere, maybe, but I just thought I’d ask.)
So, I’ve speculated that this may be a result of the HPA axis on overdrive; perpetually keeping the body in “fight or flight” mode. I know that HPA activity is upregulated in ABA, but I’m not sure if this has been directly related to the level of food suppression. I also find it interesting that running rats also exhibit changes in their sensitivity to opiate antagonists that increase with more exercise, suggesting that levels of endogenous opiates increase when these rats engage in more running behaviors. Perhaps these changes also contribute to increased motivation to engage in foraging behaviors, and they become more active during feeding and won’t just “sit down and eat their food?” I also know that some rats are more sensitive to ABA than others, and some don’t ever develop ABA. It’d be interesting to know what polymorphisms are associated with the vulnerable rats.
Any other thoughts?
What a great post and an amazing blog! Your many insights have and continue to help me a lot on the rode to recovery from my ed. I have often felt mystified by my bodies many confused hunger and satiety signals, despite being at a healthy weight now. This is of great comfort- Thank you!
I’m so glad you found this topic (and the blog as a whole—Thanks Tatyana) helpful!